Ok… I’ll try to stay calm for this one, but one day, I was in 9th grade and I was talking to my physics teacher. She said the sun was yellow, and I interrupted her, telling her that it was actually white, but for some reason, she kept saying it was yellow. It made me kinda mad, but what do you want to do? She’s the teacher: if she says she’s right, then she is (even though I knew it was incorrect). This feeling of injustice randomly came back when I was in Bordeaux today for my SAT exam, watching a video about the true color of the sun (spoiler alert: I was …).
So the sun is white, but why? Well, the real question is why would you assume it would be yellow? In fact, a lot of people know that our sky is blue because molecules of our atmosphere emit blue and absorb other colors of the spectrum. Now… I said it absorbed other colors of the spectrum, but if we assume the sun is yellow, it would only emit yellow light, so our atmosphere couldn’t be blue.
Why we believe the sun is yellow is actually because of the sunsets. The Rayleigh effect is a phenomenon that is increased during sunsets: when the white light hits ATM’s (atmosphere’s) molecules, the blue is scattered away, but yellow and orange light go through and is neither absorbed nor emitted. That’s why we see the sun as yellow. During sunsets, if you study the system sun-earth, you realise there are way more air molecules between you and the sun than when it’s up in the sky. If there are more molecules, this effect is exacerbated (because of Earth’s curvature), and we see more of the yellow-orange light from the sun and assume it is this color.
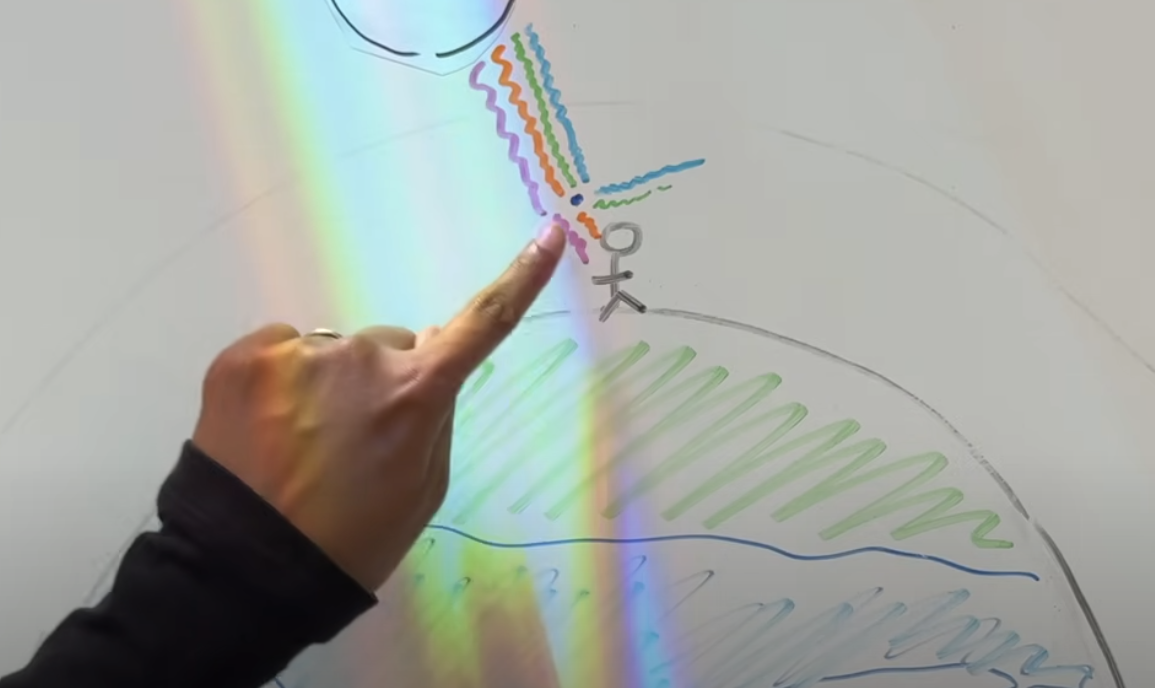
Actually, we can see the sun as a white sphere only during noon, when it’s up in the sky. This is because there are fewer air molecules between you and the sky.
Fewer molecules = decreased Rayleigh effect = sun is white
However,
Fewer molecules = less interaction between light and molecules = more light = dangerous = can’t look at the sun directly.
Ok, so the sun is white. Actually, it’s white but a little greener than “real” white. :) Ok, let’s study the black body radiation phenomenon and the “UV catastrophe”.
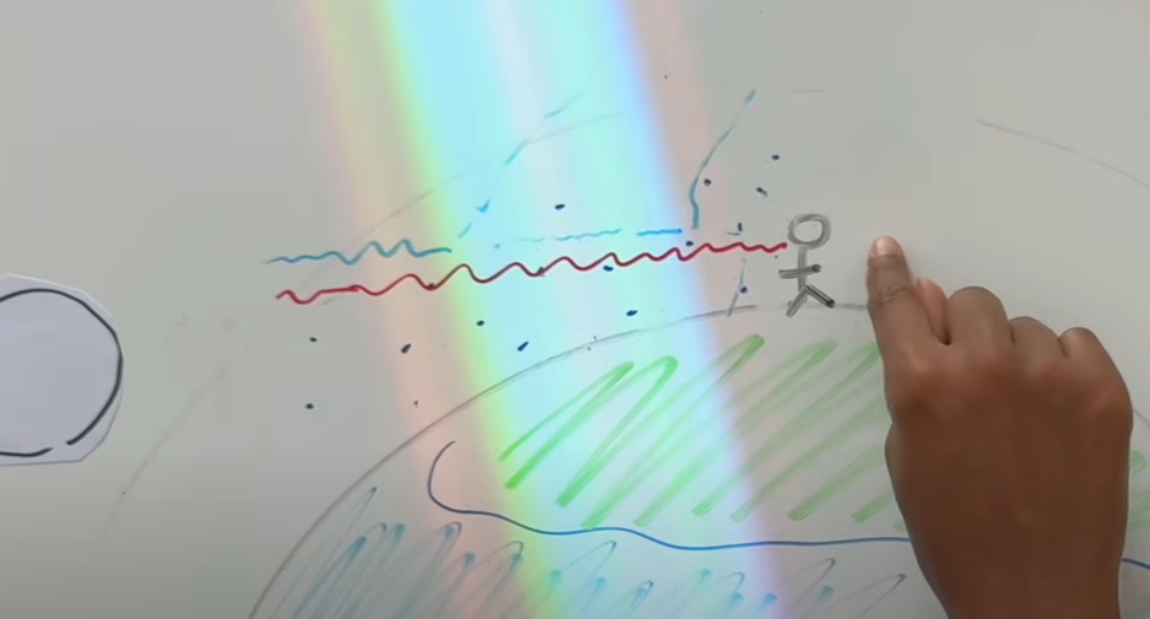
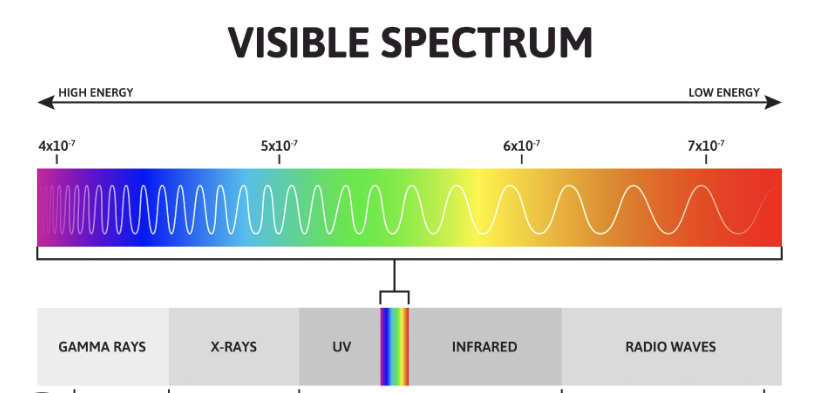
As you can see above, the hotter an object is, the more its light is going to slip into blue light. In our world, every object has a temperature, but it’s way too low to be here. That’s why we use infrared cameras: objects around us emit light but in the infrared spectrum. What is the spectrum of the sun? Well, the answer is very simple, and the theory of evolution gave us the answer: the spectrum is the visible spectrum of light! Indeed, if the sun were emitting a UV light spectrum, we would have evolved with UV light eyes; same thing for infrared light.
But here’s what traumatized an entire generation of scientists: the ultraviolet catastrophe. Imagine the sun and its molecules. The light comes from the core of the sun, travels through the molecules, and eventually gets outside of the sun to arrive at us. From here, we can say that there’s a theoretical sphere around a light wave when it’s inside the sun where it can move freely. At the second it touches the border of the sphere, it gets absorbed (because in the sun, it would get absorbed by the hydrogen molecules) and re-emitted into a different light wave. Here’s this theoretical sphere:
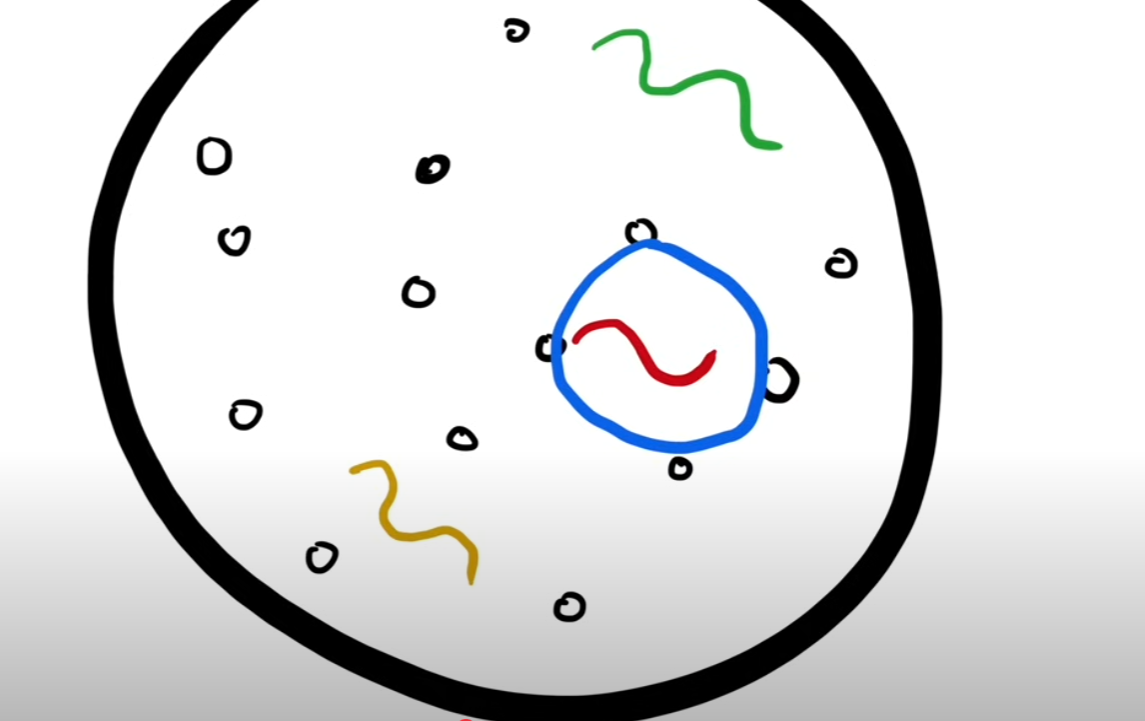
However, there’s a catch: this sphere can “withstand” only certain frequencies of light. When we study classical physics, we realise it can withstand way more UV light inside because it has a higher frequency than any other light like red light. But if this is correct, it would mean our entire world would be in the UV spectrum, which we know is not true. From that conclusion, scientists throughout the end of the 19th and beginning of the 20th century tried to explain this phenomenon, but one scientist found a solution: Max Planck.
Max Planck had the amazing idea: cutting light energy into packets (yes, that’s abstract, but no worries, we’ll get through it). First of all, why would we assume that hotter objects—more energetic objects—would be blue and less ones would be red or infrared? Well, in our previous model, we assumed that any load of energy could be absorbed and re-emitted, which is actually not true. In this model, a red light wave would get absorbed and re-emitted into 2 different colors less energetic, which would then be re-emitted into 4 other colors…
But really, each frequency and color has its own energy requirement, and that’s when we realized that red light had a smaller requirement than blue light. It’s not that red energy is, as a whole, “less energetic,” but that it can withstand more energy chunks because it only requires small energy chunks, whereas blue light requires higher energy chunks.
Statistically, you have smaller energy chunks than big ones, and this model leads to the emission of red light more than any other light color. This conclusion is opposed to the previous one stating that the theoretical sphere would only emit UV light. Indeed, even though more UV light can exist in the sphere, red light is more present because it’s less energetic.

Scientists realized that these effects combine, and it gives us: green light! Have a look at the visible light spectrum, and you’ll realise it’s in the middle between blue and red light. Actually, this is why green lasers seem more powerful than other lasers: our eyes evolved to see better green light, and when we look at a green laser, we see more green light than if we look at red light. This is also why chlorophyllous plants are green: they reject green because if they absorbed all of the spectrum, they would basically burn (white light coming from the sun would be way too powerful for photosynthesis).
Anyway, I’m glad I was right that time I said to my physics teacher the sun was white instead of yellow, and I learned a lot of things, especially about the black body effect and Planck’s work.
See you next week!
credits:
https://www.youtube.com/watch?v=P_i_brAKGfo&