Faithful to myself, last time, I was watching a YT video that I had already watched years ago about Entropy. Entropy is something weird; it’s so intuitive that it becomes counterintuitive. Sometimes, children ask parents, “Why is there time?” or “Why is the future after us and not behind us?” or even “Why do ice cubes melt?” Usually, parents, probably because of the stress that these types of questions create and because of a lack of knowledge, get angry and just say, “because it’s like that.” Well, it turns out it’s not “just like that”…
So what is entropy, really? And why is it that everything in the universe, from a sugar spill to the death of stars, seems to love falling apart? Here’s a simple analogy: you take a brand-new deck of cards. Of course, it’s perfectly ordered, and now, shuffle that deck… Boom! Instant disorder! You could shuffle it for the rest of your life, and the odds of it going back to perfect order are close to zero. That’s entropy: the universe’s tendency to move from order to disorder. Not because it “wants” to, but because statistically, disorder is way more probable. And what’s even better is that this disorder IS actually the right order, the point of balance of everything in here.
In pure physics, entropy is a measure of how much disorder or randomness exists in a system. The more disorganized or spread out the energy is, the higher the entropy (and vice versa). For example: a hot cup of coffee cools down over time because the heat spreads out into the room. Ice melts in your glass because the molecules want to move more freely.
Basically, entropy is always increasing in a closed system. And, well done, you discovered the Second Law of Thermodynamics as it is indicated by Clausius: “Heat can never pass from a colder to a warmer body without some other change, connected therewith, occurring at the same time.” If we go deeper, we explain this by the tendency of energy to spread out. In quantum physics, we call energy quanta, which are units of energy. Imagine having four atoms, each having nine quanta, touching four other atoms with two quanta. The moment they touch, quanta are exchanged, and there is a high percentage that each of the four atoms’ structures will now have six quanta each. When I say high percentage, I mean around 20%, but imagine at our scale, with trillions of billions of atoms? The chance rockets up to nearly 100%. From this point of view, we can define entropy as the homogenization of our universe. It’s the tendency of everything to go to perfect equilibrium.
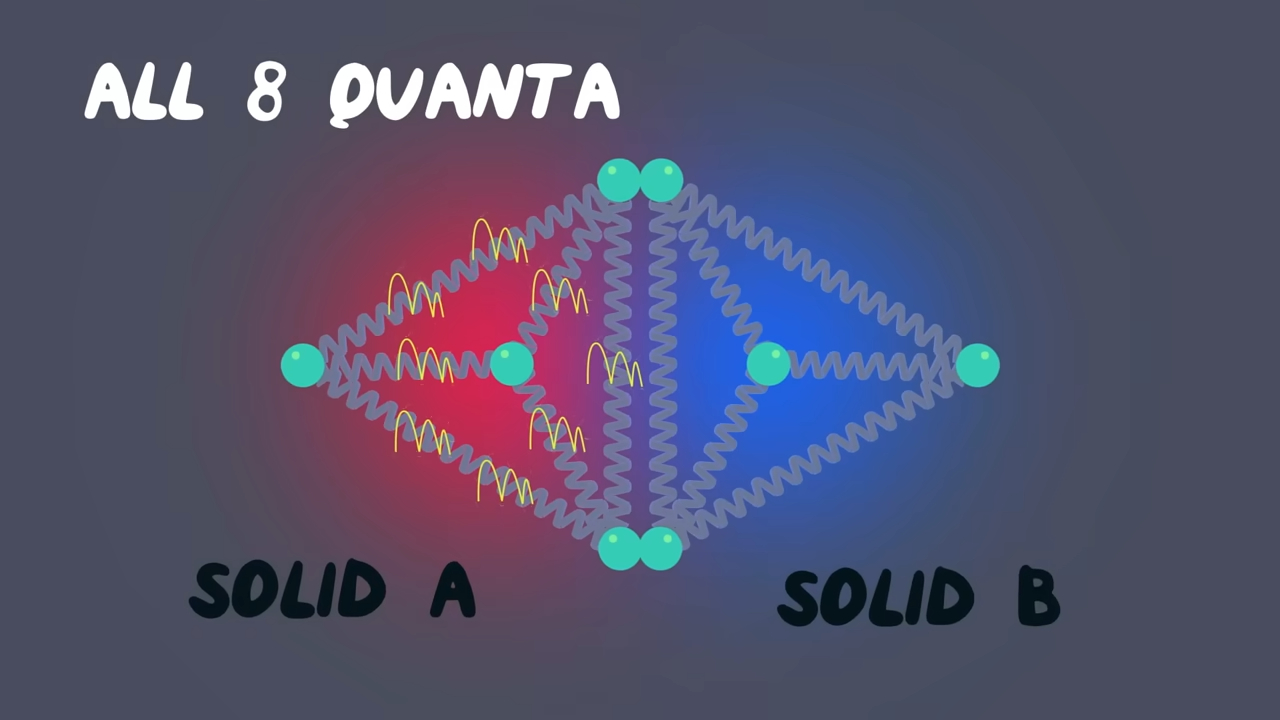
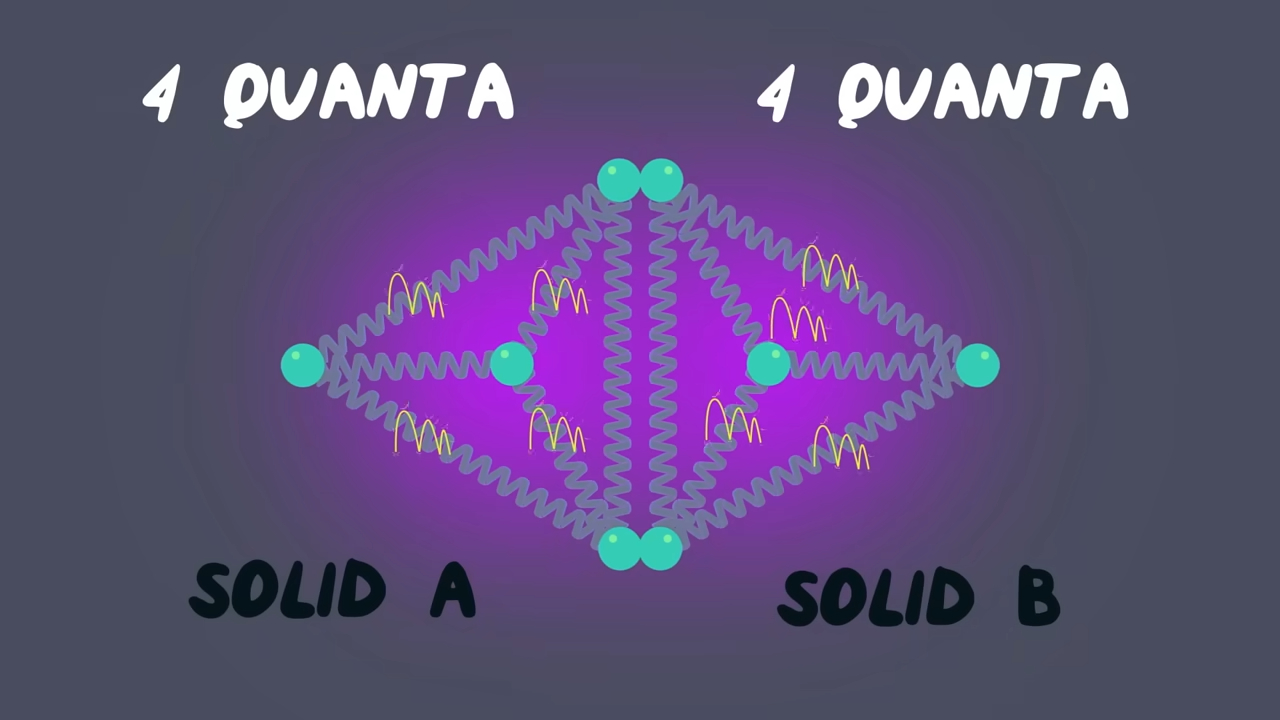
Physicists even talk about something called the “Heat Death of the Universe.” It’s this creepy and cool idea that someday, everything will be so spread out and uniform in energy that nothing interesting can ever happen again. No stars. No movement. Just… stillness. Maximum entropy. :) How funny! When you even think about it, us humans are against entropy. We organize, we structure, sometimes we even discriminate: by doing so, we think we can beat the universe, but in reality, we are not slowing entropy, not even a little. Everything, literally everything in this universe, shall remain still.
But it’s not that bad: entropy is why an egg breaks and does not un-break, a liquid gets colder and not hotter when an ice cube is placed inside, and why time travels from past to future and not in reverse. Without entropy, nothing would really even happen. That’s the end of this post… I really feel like this subject is so beautiful, so poetic. Realizing that stillness is a constant from the universe is both very tricky, scary for some, but for me, it’s only a sign of peaceful mathematical stability.
credits: https://www.youtube.com/watch?v=YM-uykVfq_E
https://www.youtube.com/watch?v=DxL2HoqLbyA&t=588s
https://en.wikipedia.org/wiki/Second_law_of_thermodynamics
